Atlas » Learn more about the world with our collection of regional and country maps. Mercury is a chemical element with the symbol Hg and atomic number 80. It is commonly known as quicksilver and was formerly named hydrargyrum (/ h aɪ ˈ d r ɑːr dʒ ər ə m / hy-DRAR-jər-əm). Atomic Number 80 Atomic Mass 200.59 Density 13.5939 g/mL. State liquid Color silvery white Electronegativity 1.8. Melting Point-38.87°C Boiling Point 356.58°C.
Atomic Number of Mercury is 80.
Chemical symbol for Mercury is Hg. Number of protons in Mercury is 80. Atomic weight of Mercury is 200.592 u or g/mol. Melting point of Mercury is -38,9 °C and its the boiling point is 356,6 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery Year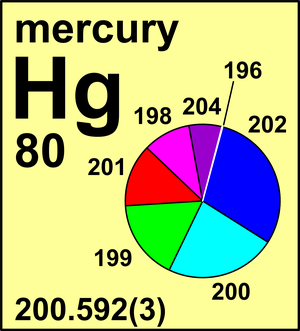
About Mercury
200 80 Hg Atomic Number
This chemical element is among the oldest known ones and the longest used by humanity. It is a shiny liquid metal, with the name given after the planet Mercury. In our body, we have micro amounts of mercury, and in excessive amounts this element is toxic to humans and animals. It is not easy to find this element uncombined, but it can be extracted from ores like mercury sulfides which are in plenty in China. Mercury is mostly used in its compounds and alloys with other metals like tin, silver, or gold, used for amalgams with such applications as dental fillings, etc. Mercury and its compounds are used for producing rectifiers, electrical equipment, fluorescent lamps, thermometers, barometers, batteries, some medical treatments, etc.
Properties of Mercury Element
| Atomic Number (Z) | 80 |
|---|---|
| Atomic Symbol | Hg |
| Group | 12 |
| Period | 6 |
| Atomic Weight | 200.592 u |
| Density | 13.5336 g/cm3 |
| Melting Point (K) | 234.43 K |
| Melting Point (℃) | -38,9 °C |
| Boiling Point (K) | 629.88 K |
| Boiling Point (℃) | 356,6 °C |
| Heat Capacity | 0.14 J/g · K |
| Abundance | 0.085 mg/kg |
| State at STP | Liquid |
| Occurrence | Primordial |
| Description | Transition metal |
| Electronegativity (Pauling) χ | 2 |
| Ionization Energy (eV) | 10.4375 |
| Atomic Radius | 150pm |
| Covalent Radius | 149pm |
| Van der Waals Radius | 155 |
| Valence Electrons | 2 |
| Year of Discovery | prehistoric |
| Discoverer | unknown |
What is the Boiling Point of Mercury?
Mercury boiling point is 356,6 °C. Boiling point of Mercury in Kelvin is 629.88 K.
What is the Melting Point of Mercury?
Mercury melting point is -38,9 °C. Melting point of Mercury in Kelvin is 234.43 K.

How Abundant is Mercury?
Abundant value of Mercury is 0.085 mg/kg.
What is the State of Mercury at Standard Temperature and Pressure (STP)?
State of Mercury is Liquid at standard temperature and pressure at 0℃ and one atmosphere pressure.
%2C_black_and_white.png?revision=2)
202 Hg Atomic Number
When was Mercury Discovered?
Mercury was discovered in prehistoric.
| Atomic Structure | |
|---|---|
| Symbol | Hg |
| Atomic Number | 80 |
| Atomic Mass | 200.6 g/mol |
| Periodic Table | |
| Group | 12 |
| Row / Period | 5 |
| Element Category | Transition dhaatu |
| Chhapa | |
Mercury ke electron shell | |
Mercury ek chemical element hae jiske symbol Hg, atomic number 80 aur atomic mass 200.6 hae.
Chhapa ke gallery[badlo | source ke badlo]
Hg Element Atomic Number
mercury mine in Idrija (Slovenia)
Mercury ore
Alchemical symbol
Amount of atmospheric mercury deposited at Fremont glacier over the last 270 years.
Liquid Mercury
Mercury 10mL
Mercury drop
Mercury drops
Rtuť - Hg
Mercury in a thermometer.
gas discharge tube with mercury
native mercury drop on cinnabarite
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||||||||||
| |||||||||||||||||||||||||||||||||||||||||
Hg 2 Atomic Number
