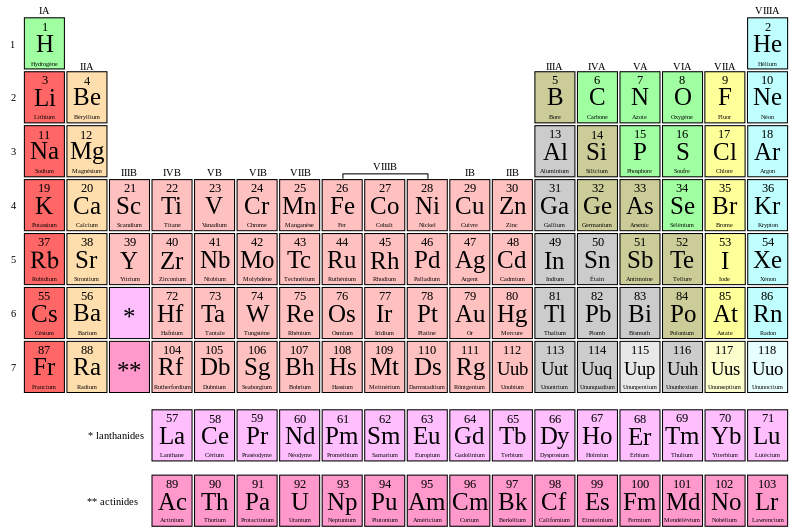
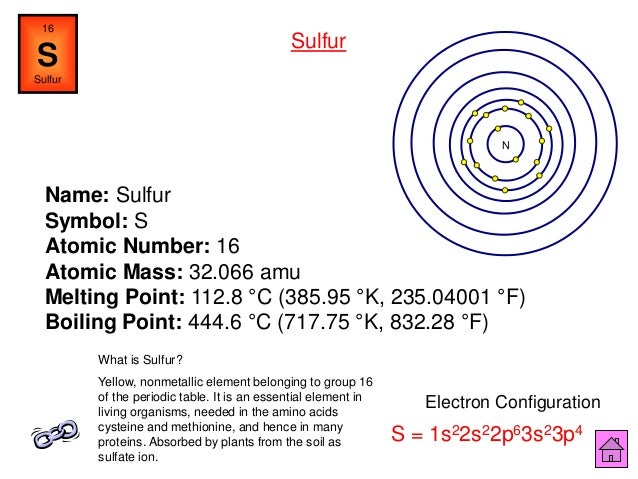
It seems like it must be your homework. Dlna servers for mac os. So, if u didn't pay attention in the class try to from the next time as science is fun. So, coming back to your question the 1st thing you should do is 1- Pay attention to the given atomic number. Atomic number 16 - an abundant tasteless odorless multivalent nonmetallic element; best known in yellow crystals; occurs in many sulphide and sulphate minerals and even in native form (especially in volcanic regions) sulfur, sulphur, S. Chemical element, element - any of the more than 100 known substances (of which 92 occur naturally) that cannot be separated into simpler substances. Bash profile for mac.
Also found in: Thesaurus.
| Noun | 1. | atomic number 16 - an abundant tasteless odorless multivalent nonmetallic element; best known in yellow crystals; occurs in many sulphide and sulphate minerals and even in native form (especially in volcanic regions) sulfur, sulphur, S chemical element, element - any of the more than 100 known substances (of which 92 occur naturally) that cannot be separated into simpler substances and that singly or in combination constitute all matter brimstone, native sulfur, native sulphur - an old name for sulfur sulfide, sulphide - a compound of sulphur and some other element that is more electropositive oil of vitriol, sulfuric acid, sulphuric acid, vitriol - (H2SO4) a highly corrosive acid made from sulfur dioxide; widely used in the chemical industry |
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.

Link to this page:
Atomic Number Chart
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
Atomic Number 161
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
Photoshop for mac бесплатно. The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
